Neuralink's approach involves implanting electrodes directly into the brain, where they're tasked with interpreting signals and stimulating brain cells in an effort to restore movement and sight, and, according to Musk, allow humans to merge with AI.May 17, 2023
Your guide to a better future
The Bionic Eye That Could Restore Vision (and Put Humans in the Matrix)
We could be able to manipulate our own reality.


Inside the front door at Science Corp. in Alameda, California, lies a brightly lit room with large, transparent windows. On a late November afternoon, three gowned surgeons carefully circle a New Zealand white rabbit laid out on an ocean-blue cloth. About a month prior, the rabbit — named Leela, after Futurama's one-eyed heroine — received an injection through the white of her eyeball.
Just outside the surgery room, Max Hodak, the CEO of Science Corp., stands in jeans and a black hoodie, cradling a laptop in the crook of his arm. The presentation on his screen shows a small device, about the size of a penny, attached to a thin tail of wiring. It's a device he hopes can restore a critical sense and help the blind see again. It doesn't look like much — a miniature city of electronics attached to a microLED display just 2mm square — but it doesn't have to.
The prosthesis he's showing off is known as the Science Eye, and once it's been proved safe and effective, it'll be implanted on top of, and inside, the eyeballs of human patients suffering from diseases where the eye's light-sensing cells have died. The idea is to coax other cells within the eye to receive and translate light signals. The device was unveiled as the biotech exited stealth on Nov. 21 last year.
It had been a busy morning for Hodak, but an air of quiet optimism suffused the Science Corp. facility during my visit. In the months since, the company's first scientific paper was uploaded to bioRxiv, a repository for preprint scientific articles, describing the extensive foundational work Science Corp. has undertaken, including demonstrating how its technology works in rabbits like Leela, and readying it for future trials to test its vision-restoring capabilities.
As I stand with Hodak outside the surgery, he runs through images on his laptop, pointing out the form factor of the Science Eye and how many pixels the team has been able to jam into the device's wafer-thin microLED. The number stands at an impressive 16,000, allowing for a resolution he says is about "eight times better than an iPhone 13." He shows off a brief demo of the kind of "vision" someone with a Science Eye might have. Red pixels dance around a screen, recapitulating a view of a street and a human waving their hands.
The microLED device, which Science Corp. calls FlexLED, is just one component of the Science Eye. For it to restore even this form of vision to patients, the Science Corp. team first needs to deliver a gene to a specific region of the eye and demonstrate it can generate electrical signals in the regions of the brain responsible for controlling sight. That's where Leela comes in.
While Hodak and co-founder Alan Mardinly explain the process to me, behind them, Leela's eyeball is carefully being removed from its socket.
TO READ THIS article, your eye and your brain are involved in a frantic dance, enlivened by a storm of light and electrical signals. This dance, honed by millions of years of evolution, provides us with the sensation of sight.
Light from your screen is focused by the lens of your eye onto the retina, a layer of tissue at the rear of the eye containing light-sensing cells known as photoreceptors. These cells, which are shaped like rods and cones, contain molecules known as opsins, which can convert the incoming light into an electrical signal.
That signal is eventually passed forward to nerve cells called retinal ganglion cells, which snake from the eye up into the brain as the optic nerve, transmitting information that creates a visual image of the world.
In genetic diseases, such as retinitis pigmentosa and age-related macular degeneration, abnormalities in the layer of photoreceptors in the retina ultimately lead to their death. With the photoreceptors lost, light signals can no longer be translated to electrical signals, resulting in blindness. It's not a perfect analogy, but think of the eye as a house. There's still electricity flowing into the wires of the home, but with these diseases, all the lightbulbs have blown out.
The FlexLED sits at the back of the eye, while the electronics package sits on top of the eyeball in the same way a glaucoma shunt might.
Zooey Liao/CNETFortunately, there are other ways to light it up. While the photoreceptors are lost in retinitis pigmentosa, the RGCs — and other cells in the retina — remain intact. The brain can still decode light signals. The idea behind the Science Eye is to modify these RGCs to become photoreceptive so they can be stimulated, by light, and send those signals to the brain. It's like bringing lamps into the house and plugging them in to provide light.
The modification requires an injection of a specially designed opsin, which has been genetically manipulated and encased in a deactivated virus to seek out RGCs. The Science Corp. team has been able to show that the opsin makes its way to RGCs, in experiments with neurons derived from stem cells and in retinal organoids, simulacra of a human retina. In short, they can illuminate the house with lamps, rather than lightbulbs.
"What we want to do is test it in an adult human ... but we can't until we're allowed to," says Mardinly, the co-founder and director of biology. "The next best thing is to grow a retina and test it in those human cells."
The organoids, which develop from stem cells into a mixture of cells including RGCs, are doused in a solution of the viral construct containing opsins. About 10 weeks later they're placed under a microscope, where researchers, including cell engineer Kevin Smith, go searching for bright red cells — signifying that the opsin has landed in the organoid's RGCs. I'm told this is working well, with about one in five RGCs expressing the construct designed by Science Corp. Further refinement of the viral construct and the opsin is expected to deliver even better expression.
This work at the lab bench shows that the method works in vitro, outside of a living organism. But what about inside a living organism? For that, the company needs Leela. More specifically, it needs her eyes.
AS I WANDER through the laboratories at Science Corp. with Hodak and Mardinly, I pass by scientist Amy Rochford as she works with tweezers and a paintbrush to delicately manipulate a thumbnail-size piece of tissue.
This, she tells me, is Leela's eyeball.
Rochford slices it open, removing various parts of the eye, like the lens and the vitreous, a gel-like layer in the middle, before spreading the ocular orb open like a flower with four petals. The paintbrush helps access the retina and delicately sections it out for processing, so another member of the Science Corp. team — someone like cell engineer Smith — can study it down the lens of a microscope.
Rabbit eyes aren't quite the same as human eyes. One of the chief differences is a region known as the fovea, a central depression in the retina where a lot of light-sensitive cones are packed tightly together. Rabbits have a streak of cells whereas humans have a pit, Mardinly notes, and the fundamental eye biology is a little different, but the New Zealand Whites provide a great starting point for this kind of research.
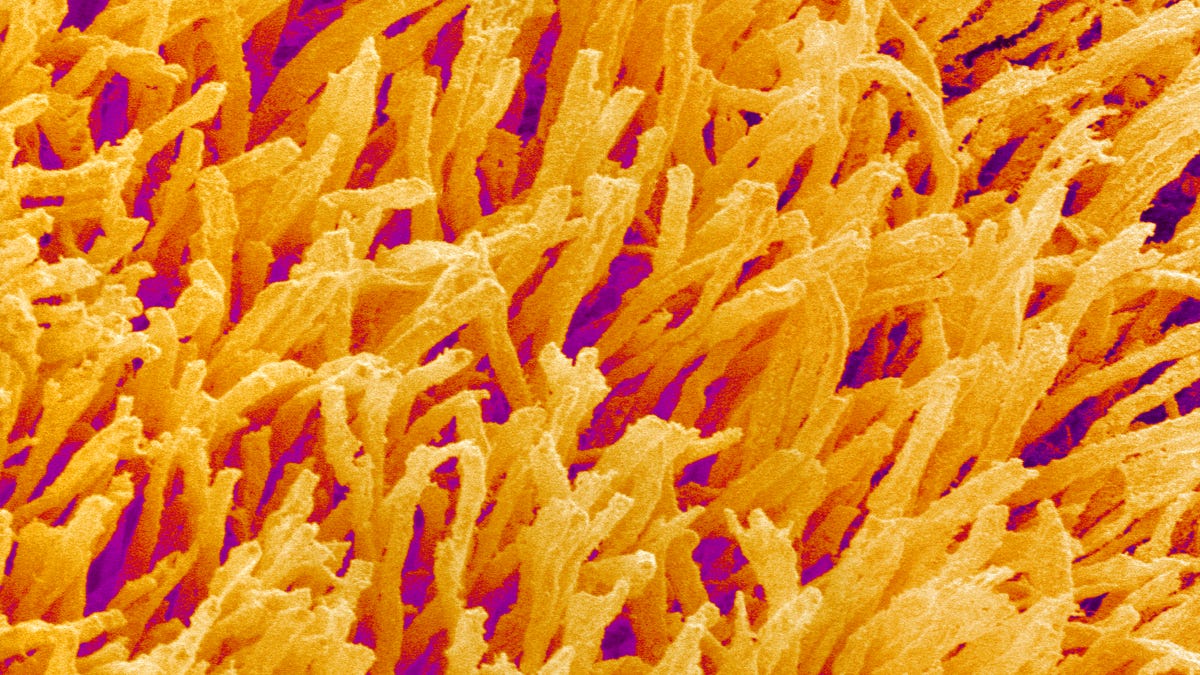
The rods and cones in a rabbit's retina at 4,000x magnification.
Getty ImagesScience Corp. needs to validate two concepts. First, its viral construct, containing the opsin, has to get into RGCs in the rabbit retina. Second, the pulsing light of the FlexLED device needs to stimulate the opsins and send signals to the brain. In rabbits, the company isn't trying to restore vision just yet. Rather, it's doing the basic science to show the method works.
Early results indicate it does. Experiments with two rabbits, described in a preprint the team released in February, show they've been able to make RGCs light-sensitive. They've also been able to pulse the FlexLED device and detect activity in the visual centers of the brain.
However, to stimulate the opsin in RGCs, patients (including Science's rabbits) need to be exposed to a specific wavelength of light. The opsin doesn't respond to natural light like the human eye; it's unable to generate a full picture of the environment like healthy photoreceptor cells can. For that reason, the Eye will require patients to wear a pair of glasses with cameras that communicate information, wirelessly via infrared, to the FlexLED implanted over the retina.
The vision restoration for early patients won't be a miraculous return to 20/20, but it will help them make sense of their world; the sensation will be akin to sight but with much less fidelity.
To restore high-resolution vision, there are physiological barriers that have yet to be overcome. For instance, the human retina contains more than 100 million photoreceptors in each eye, but only about 1 million RGCs, a difference that's hard to overcome — but not impossible. In some ways, it might even be considered easier to stimulate just the RGCs and get them to fire.
Patients would be required to wear glasses that communicate wirelessly with the Science Eye implant.
Zooey Liao/CNETRGCs are also separated into distinct types, which relay slightly different information to the brain. "I've heard people refer to them as, like, Photoshop filters," Hodak explains. "When you stack them all together, you get the natural scene."
In theory, a future version of the FlexLED device could drive different types of RGCs. Hodak notes that he isn't sure if this is possible just yet, but with refinement, the device may even be able to have a constant, one-to-one mapping between a pixel on its FlexLED screen and an individual RGC. Combined with the brain's ability to adapt over time, high-resolution vision restoration could be within reach.
Read more: Virtual Exams Are the Future of Eye Care
SCIENCE CORP. ISN'T the only team working on modifying the eye to restore vision, but in the burgeoning field of optogenetics, its approach is unique.
A number of companies are experimenting with different techniques, including using gene-editing RGCs and light-altering goggles. For instance, French biotech company GenSight is working on a similar optogenetics system, using gene therapy and glasses. Its system doesn't require overlaying a thin microLED on the retina like the Science Eye, making it less risky. Instead, it uses goggles to amplify the ambient, natural light into a monochromatic signal that genetically edited RGCs can decipher.
This method arguably provides less fine control of opsin activation in the RGCs, but it's already in clinical trials and has been shown to "partially restore" vision in a patient with retinitis pigmentosa, according to a 2021 paper in the journal Nature. The patient was able to detect objects, like a notepad on a table, after wearing the GenSight glasses over a matter of months.
Hodak's previous place of employment, Neuralink, also recently entered the race to restore vision. Hodak co-founded that brain-computer startup with a team of scientists, engineers and, yes, Elon Musk back in 2016. Hodak left in 2021. The comparison between the two companies has been the primary focus of articles regarding Science Corp. to date, with publications dubbing the biotech firm a "Neuralink rival."
Comparing the two is, at this stage, kind of like comparing In-N-Out Burger to Whole Foods. Neuralink's approach involves implanting electrodes directly into the brain, where they're tasked with interpreting signals and stimulating brain cells in an effort to restore movement and sight, and, according to Musk, allow humans to merge with AI.
Regulatory bodies are already showing concerns about that approach. Neuralink has failed one application to the US Food and Drug Administration to get its product into trials. Science Corp. will face the same hurdles, but the Science Eye has one major advantage over the Link: Surgery of the eye comes with its own dangers, but they're not comparable to inserting electrodes into the brain.
Safety remains paramount, however. Raymond Wong, a stem cell biologist at the University of Melbourne working on eye disease treatments, notes Science will need to make sure "the implant doesn't cause damage to neighboring retina cells, increase intraocular pressure [or] cause intraocular inflammation." These are potential problems Hodak, Mardinly and others are trying to solve in preclinical work using rabbits like Leela, and likely primates too, but the real test will come when the devices first make their way into humans.
That moment will, Hodak hopes, be just the beginning. Though the Science Eye is the only device that's been publicly unveiled, it's clear Science Corp. is already thinking beyond. It would be shortsighted to assume otherwise. After all, the startup's ambitions are lurking right there in the name. Science. This isn't a company built around one product or goal.
"It's early days but if this works it'll be an enormous company," says Hodak. "Like, we're not in any one area of medical devices, we might not even be just medical devices eventually." Hodak is coy when pressed about those devices, saying they'll be announced if and when they're ready. There have been some indications, though, of Science Corp.'s ultimate ambitions.
WHEN HODAK ANNOUNCED his new venture on his personal blog in late 2021, he made a bold proclamation. "The future isn't better smartphones or AR glasses: It's making the sensorium itself directly programmable, and maybe even adding new senses entirely," he wrote.
This idea — reprogramming the brain to experience new senses — isn't limited to the realm of science fiction. The brain is intrinsically linked to how we experience the world. We've evolved five senses, at least according to Aristotle, and that view holds true today: touch, smell, sight, taste and hearing. Modern science has added a couple more. Our balance is a special sense, as is proprioception, the ability to discern our body's location and movement.
There are even scientists who believe the number of senses we have stretches into the 20s; our ability to discern the passage of time and our body's reaction to hot and cold states are other senses. What Hodak seems to be getting at when he talks about programming the sensorium is the notion that the brain isn't an unchanging organ. It's receptive to new, external inputs and, over time, it can learn to adapt to them. Give it a new way to interact with the world, and slowly it will process that information in a way the body can understand.
Now, instead of restoring vision, perhaps imagine a Science Eye implanted in a person with perfect vision. It might stimulate the brain in such a way that the person sees specific images or places, via fine control of the RGCs. You could see and interact with an entire world that isn't there. It's kind of like plugging in to a simulation, a virtual world plugged directly in to your eye. It's an idea exemplified by the posters that line the hallways of Science Corp., artwork jokingly referred to as "propaganda" by Hodak. One, in particular, catches my eye. It's an abstract piece featuring a series of colored nodes in the shape of a brain. Underneath it reads: "Alter the brain, alter reality."

Announcing Science Corp, Hodak signed off with, "See you in the Matrix."
Getty ImagesIf that reminds you of a now classic science fiction film, that's deliberate. Hodak signed off from his announcement blog post with the words: "See you in the Matrix."
In that short sentence, perhaps, we get a glimpse of what Science Corp. intends to accomplish.
We're a long way from that future. And, to be clear, I didn't find any locked doors during my tour of the Science Corp. facility in Alameda. There was no suggestion that secretive plans were taking shape behind the scenes -- to supercharge our senses or create artificial worlds where you can upload karate skills directly into your brain. But, as the company exited stealth, that blog post from Hodak was at the front of my mind. So were the rabbits.
Before I leave, animal technician Jess Tapp takes me into Science Corp.'s animal house where rabbits hide out in their hutches. She knows them by name and speaks to them like they're her personal companions. One of the rabbits — I didn't write down the name — is rather shy. As I bend down and look inside, I can see her nose twitching a little, her ears at attention.
She bounds, carefully and inquisitively, to the front of the hutch. As she does so, the light catches in her eyes, reflecting the deep red typical of her breed. The storyteller in me hopes to see something in them, but the rabbit just turns and heads to safety at the back of her hutch.
Disclosure: Kevin Smith, Science Corp.'s cell engineer, is a childhood friend of Jackson Ryan.



No comments:
Post a Comment