Shanghai starts administering inhalable COVID-19 vaccine

"East China's Shanghai has started administering an inhalable COVID-19 vaccine as a booster dose. /CanSino Biologics
East China's Shanghai has started administering an inhalable COVID-19 vaccine as a booster dose. /CanSino Biologics
East China's Shanghai has started administering an inhalable COVID-19 vaccine as a booster dose from Wednesday, according to the Information Office of Shanghai Municipality.
Developed by Chinese company CanSino Biologics, the aerosolized adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) provides a non-invasive option. It uses a nebulizer to change liquid into aerosol for inhalation through the mouth, according to the company.

The aerosolized adenovirus type-5 vector-based COVID-19 vaccine. /CanSino Biologics
The aerosolized adenovirus type-5 vector-based COVID-19 vaccine. /CanSino Biologics
The company said on its official WeChat account that the vaccine can "effectively induce comprehensive immune protection in response to SARS-CoV-2 after just one breath."
It can "induce strong humoral, cellular and mucosal immunity to achieve triple protection," the company added.
Compared with intramuscular injection, the inhalable vaccine requires only one-fifth of its dosage. It can only be used as a booster dose for now, and adults aged 18 years and above can choose this vaccine as an alternative.
Read More:
Chinese inhalable COVID-19 booster vaccine safe and effective
Inhaled delivery of a potent neutralizing nanobody effective against SARS-CoV-2
A recent study by researchers from China, available on the bioRxiv* preprint server, shows how stable neutralizing nanobody Nb11-59 might be a promising prophylactic and therapeutic molecule against coronavirus disease (COVID-19) – especially if administered via the inhalatory route.
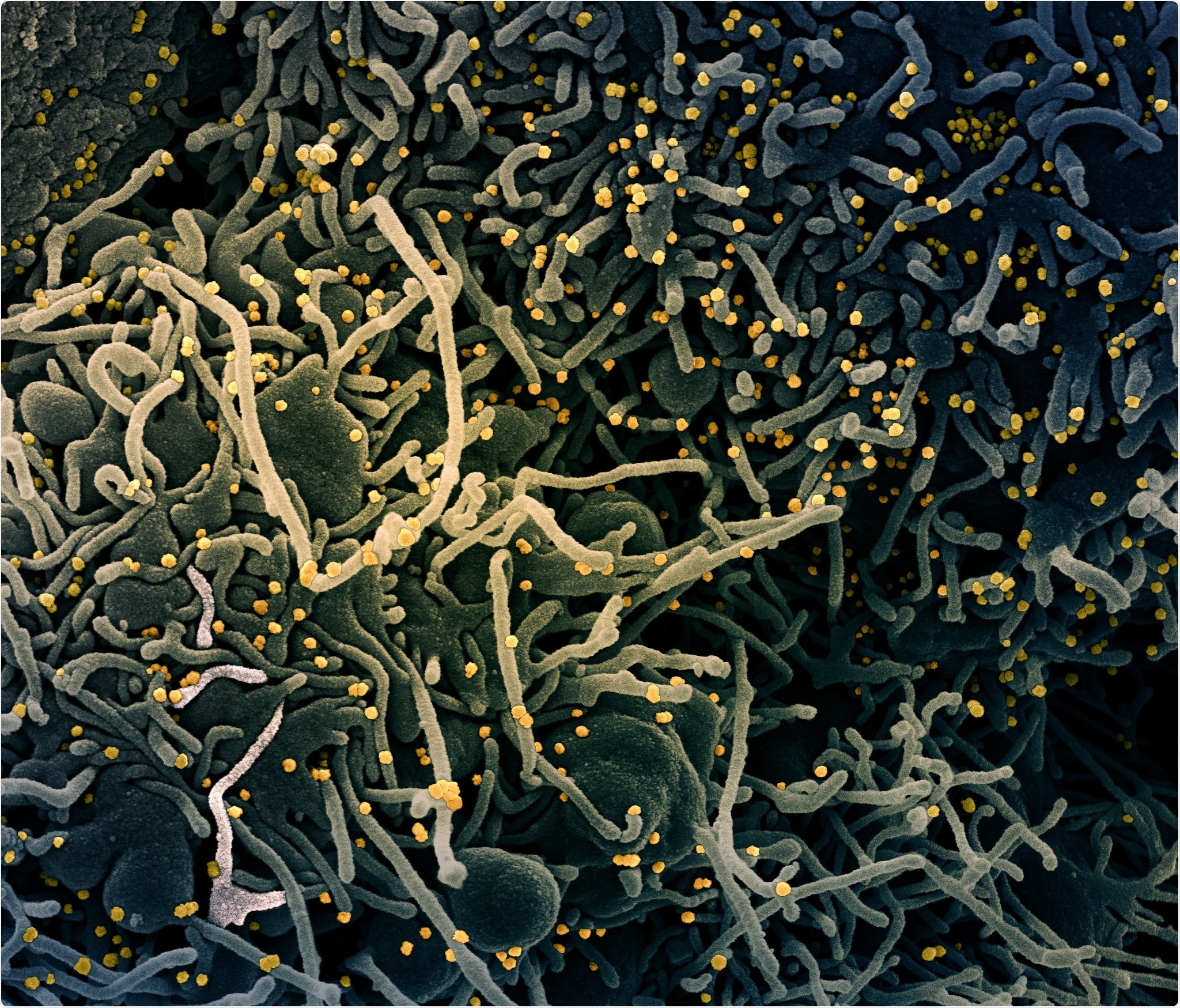
Colorized scanning electron micrograph of a VERO E6 cell (blue-green) exhibiting elongated cell projections and signs of apoptosis, after infection with SARS-COV-2 virus particles (yellow), which were isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
The outbreak of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as an unprecedented global pandemic, urging rapid development of effective prophylactic or therapeutic solutions. Thus far, this pursuit was elusive, but several candidates showed promise in preclinical trials.
Akin to other coronaviruses, the spike glycoprotein homotrimer on the SARS-CoV-2 plays a significant role in receptor binding and viral entry. Its S1 subunit harbors the C-terminal receptor-binding domain (RBD), which binds to the human angiotensin-converting enzyme 2 (ACE2) present on our cells.
Consequently, biologic drugs that aim to neutralize SARS-CoV-2-RBD/ACE2 interaction are growing explosively, recognizing this event as an Achilles heel of the whole infection process. One of the salient research directions is to construct COVID-19-targeting antibodies that exhibit broad-spectrum activity against both the wild-type and the mutant viral strains.
In addition to conventional monoclonal antibodies, heavy-chain-only antibodies isolated from camels (also known as single-domain antibodies or nanobodies) provide a paradigm shift in the development of therapeutic antibodies. Also, novel delivery strategies of antibodies should be explored and encouraged.
Taking all that into account, a group of scientists from Shanghai Novamab Biopharmaceuticals Co., Shanghai University of Medicine and Health Sciences, Chinese Academy of Sciences, and China Pharmaceutical University in Nanjing, identified stable nanobodies that successfully recognize SARS-CoV-2 RBD – including several mutants.
A roadmap towards effective nanobodies
In this study, the researchers report nanobody phage display libraries derived from four camels which were immunized with the SARS-CoV-2 spike RBD, and from which 381 nanobodies were identified to recognize the RBD – including the eight variants of SARS-CoV-2.
Considering the RNA viral nature of SARS-CoV-2, as well as the reported mutations of SARS-CoV-2-RBD in various countries, cross-activity of 381 nanobodies against various RBD mutants has also been studied through periplasmic extract enzyme-linked immunosorbent assay.
The Biolayer Interferometry based assay was carried out in order to measure the binding kinetic of nanobodies to RBD, while their neutralizing potential was appraised with the use of plaque reduction neutralization test (PRNT).
Finally, a high-efficiency expression system was established in specifically selected yeast organism. Formulated nanobodies were examined for their purity by size exclusion chromatography (SEC-HPLC), and their nebulization was conducted by employing PARI eFlow rapid Nebulizer System.
A robust blocking activity of Nb11-59
"Herein, we showed that 7 RBD-directed nanobodies identified were capable of blocking the interaction between ACE2 and the eight RBD-mutant variants, with Nb11-59 showing the most potent blocking activities", study authors summarize their main findings.
The observed Nb11-59 successfully recognized the wild-type and eight kinds of RBD protein mutants. Furthermore, this specific nanobody was efficient in laboratory inhibition of the replication cycle of authentic SARS-CoV-2, with 50% neutralization dose of 0.55 μg/mL – which is similar or lower than values for several monoclonal antibodies isolated from human B cells.
Likewise, a sufficient blocking activity was also demonstrated against SARS-CoV-1 (i.e., a causative agent of the original SARS outbreak) and its close relative, bat SARS-like coronavirus WIV1 (found in Chinese rufous horseshoe bats).
The encouraging fact is that Nb11-59 can be mass-produced on a large-scale using Pichia pastoris (yeast species for the production of recombinant proteins) with 99.36% purity. The nanobody also demonstrated adequate stability profile, while nebulization did not influence its stability.
The advantages of single domain antibodies
Taking into account their biophysical traits and rather small size, single domain antibodies have a myriad of advantages in the treatment of respiratory tract infections. As already mentioned, they can be produced on a large scale and at a very low cost.
"We showed that Nb11-59 expression could reach 20 g/L through fermentation using the yeast expression system, which means that it could be rapidly and widely used as a preventive or therapeutic molecule", emphasize study authors in their bioRxiv paper.
Moreover, Nb11-59 (as a single domain antibody with small size) could be delivered to the infection site via inhalation, reinforced by its high stability at the temperature range between 4 and 40 °C, but also its consistent post-nebulization stability profile.
Due to the highly infectious COVID-19 pandemic, such inhaled delivery of Nb11-59 may likely be effective in controlling the spread of SARS-CoV-2. This novel way of drug delivery may offer more favorable drug administration to patients but also improved drug absorption effects.
In conclusion, this paper represents a creative endeavor to find the first nanobody drug against SARS-CoV-2 in respiratory form. Such a promising therapeutic molecule is definitely worthy of further research in order to accelerate its clinical development and potential real-world implementation.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Gai, J. et al. (2020). A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. bioRxiv. https://www.biorxiv.org/content/10.1101/2020.08.09.242867v1




.jpeg)

.jpeg)
.png)
.jpeg)
.jpg)



