Like CRISPR-based systems, the Fanzor system can be easily reprogrammed to target specific genome sites, and Zhang said it could one day be developed into a powerful new genome editing technology for research and therapeutic applications. The abundance of RNA-guided endonucleases like Fanzors further expands the number of OMEGA systems known across kingdoms of life and suggests that there are more yet to be found.
A Cryo-EM map of a Fanzor protein (gray, yellow, light blue, and pink) in complex with wRNA (purple) and its target DNA (red). Non-target DNA strand in blue. (Courtesy of the Zhang lab)
June 28, 2023 11:31 AM EDT Updated 02:55 PM R&D
Meet ‘Fanzor,’ a cousin of CRISPR and Feng Zhang’s new gene editing tool
Ryan Cross Senior Science Correspondent
Two independent teams, both led by scientists from the Massachusetts Institute of Technology, unveiled new gene editing tools on Wednesday based on proteins called “Fanzors” that are found in eukaryotes, a broad branch on the tree of life that includes algae, fungi, plants, and animals.. . "
Researchers uncover new CRISPR-like system in animals that can edit the human genome
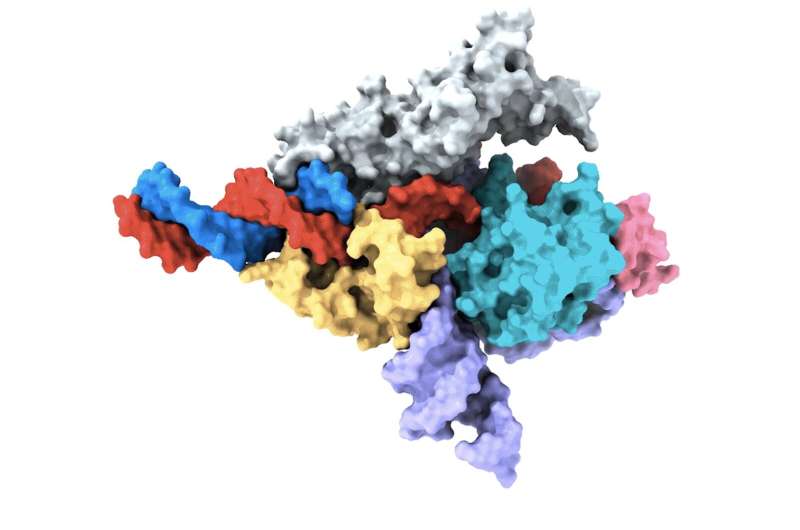
In a study published in Nature, the team describes how the system is based on a protein called Fanzor.
- They showed that Fanzor proteins use RNA as a guide to target DNA precisely, and that Fanzors can be reprogrammed to edit the genome of human cells.
- The compact Fanzor systems have the potential to be more easily delivered to cells and tissues as therapeutics than CRISPR/Cas systems, and further refinements to improve their targeting efficiency could make them a valuable new technology for human genome editing.
"CRISPR-based systems are widely used and powerful because they can be easily reprogrammed to target different sites in the genome," said Zhang, senior author on the study and a core institute member at the Broad, an investigator at MIT's McGovern Institute, the James and Patricia Poitras Professor of Neuroscience at MIT, and a Howard Hughes Medical Institute investigator. "This new system is another way to make precise changes in human cells, complementing the genome editing tools we already have."
Searching the domains of life
A major aim of the Zhang lab is to develop genetic medicines using systems that can modulate human cells by targeting specific genes and processes.
"A number of years ago, we started to ask, 'What is there beyond CRISPR, and are there other RNA-programmable systems out there in nature?'" said Zhang.
- Two years ago, Zhang lab members discovered a class of RNA-programmable systems in prokaryotes called OMEGAs, which are often linked with transposable elements, or "jumping genes," in bacterial genomes and likely gave rise to CRISPR/Cas systems.
- That work also highlighted similarities between prokaryotic OMEGA systems and Fanzor proteins in eukaryotes, suggesting that the Fanzor enzymes might also use an RNA-guided mechanism to target and cut DNA.
- Co-first author Makoto Saito of the Zhang lab led the biochemical characterization of the Fanzor proteins, showing that they are DNA-cutting endonuclease enzymes that use nearby non-coding RNAs known as ωRNAs to target particular sites in the genome.
It is the first time this mechanism has been found in eukaryotes, such as animals.
Unlike CRISPR proteins, Fanzor enzymes are encoded in the eukaryotic genome within transposable elements and the team's phylogenetic analysis suggests that the Fanzor genes have migrated from bacteria to eukaryotes through so-called horizontal gene transfer.
"These OMEGA systems are more ancestral to CRISPR and they are among the most abundant proteins on the planet, so it makes sense that they have been able to hop back and forth between prokaryotes and eukaryotes," said Saito.
To explore Fanzor's potential as a genome editing tool, the researchers demonstrated that it can generate insertions and deletions at targeted genome sites within human cells.
- The researchers found the Fanzor system to initially be less efficient at snipping DNA than CRISPR/Cas systems, but by systematic engineering, they introduced a combination of mutations into the protein that increased its activity 10-fold.
- Additionally, unlike some CRISPR systems and the OMEGA protein TnpB, the team found that a fungal-derived Fanzor protein did not exhibit "collateral activity," where an RNA-guided enzyme cleaves its DNA target as well as degrading nearby DNA or RNA.
- The results suggest that Fanzors could potentially be developed as efficient genome editors.
- Fanzor shares structural similarities with its prokaryotic counterpart CRISPR-Cas12 protein, but the interaction between the ωRNA and the catalytic domains of Fanzor is more extensive, suggesting that the ωRNA might play a role in the catalytic reactions. "We are excited about these structural insights for helping us further engineer and optimize Fanzor for improved efficiency and precision as a genome editor," said Xu.
"Nature is amazing. There's so much diversity," said Zhang. "There are probably more RNA-programmable systems out there, and we're continuing to explore and will hopefully discover more."
More information: Fanzor is a eukaryotic programmable RNA-guided endonuclease, Nature (2023). DOI: 10.1038/s41586-023-06356-2
Journal information: Nature
Provided by Broad Institute of MIT and Harvard



No comments:
Post a Comment